What Is A Breast Augmentation?
A breast augmentation, also known as an augmentation mammaplasty or a breast enlargement, is a cosmetic surgery that increases breast size or fixes breast asymmetry. Breast augmentation enlarges breasts using implants or by fat transplantation. Unless done in conjunction, augmentation mammaplasty is not a substitute for a mastopexy, which is a breast lift that corrects significant sagging.

Who Are The Ideal Candidates For Breast Augmentation Surgery?
Breast enlargement candidates include women that are in good physical and mental health and have fully developed breasts. However, age or even pregnancy has made you feel less confident about them. Patients interested in having a breast augmentation done usually want to:
- Fix asymmetrical breasts
- Increase their breasts size
- Restore lost breast volume from pregnancy
- Restore lost breast volume from significant weight loss
"I had breast augmentation with liposuction with Dr. Hunsaker and I'm very happy with my results, he did a great job. He is a professional plastic surgeon and his staff is very friendly. They were more than willing to answer any questions or concerns I had. I would definitely recommend anyone to go see him!"
Breast Implant Sizes

How Is Breast Augmentation Surgery Performed?

There are two types of breast augmentation procedures. The first method is by using implants. Implant options include silicone, saline, "gummy bear," round, smooth and textured. During your procedure, Dr. Hunsaker will insert your chosen implant underneath either breast tissue or the chest-wall muscle.
We are now offering the new Mentor Xtra and Boost profile silicone gel implants! These implants are form-stable meaning the shape of the breast holds for more upper pole fullness.
The second method is by fat transplantation or fat transfer. This method uses liposuction to harvest excess fat from other parts of the patient's body. Then, Dr. Hunsaker injects the fat into the breasts. With either procedure, surgery will take about 60 - 90 minutes.
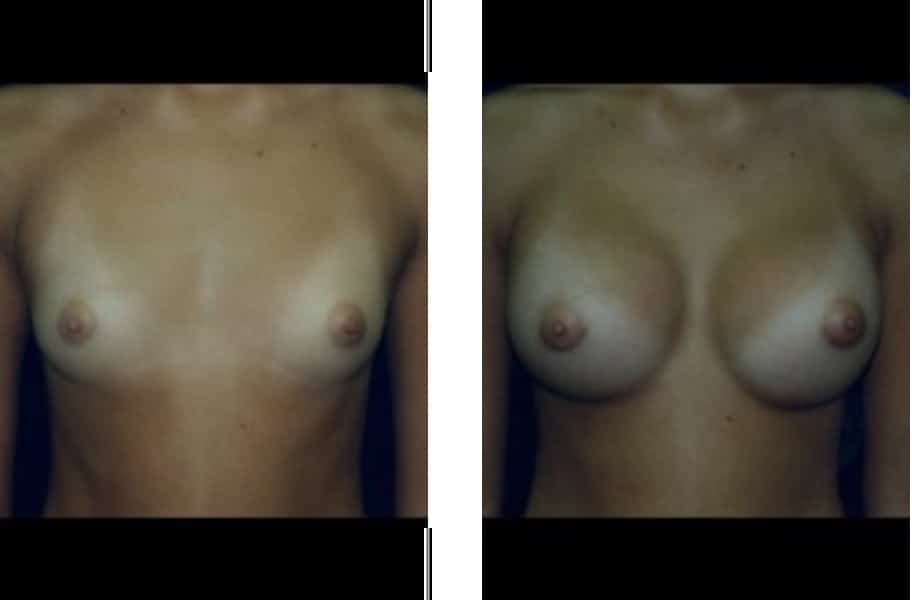
Breast Augmentation Before & After Photos:
What Is The Recovery From Breast Augmentation Surgery?

Are There Risks To Breast Augmentation Surgery?
In addition to the risks associated with any medical procedure and anesthesia, there are risks related to a traditional breast augmentation. With the location of the surgery, the risks could involve loss of sensation in the breast or nipple area, but this is not common. The most common risks of breast augmentation include:
- Asymmetry
- Capsular contracture
- Fat transfer calcification
- Implant leaks or deflation
- Irregularities in breast contour/shape
- Loss of volume after fat transfer
- Partial or total loss of nipple/areola
- Temporary or permanent change in nipple/breast sensation

What Can I Expect During Breast Augmentation Recovery?
In general, when we discuss breast augmentation recovery, we're talking about the time it takes to get back to work and other light activities. That said, your recovery will have multiple stages. These include:
- The Day of Surgery. Breast augmentation is an outpatient procedure. You'll be released to a loved one once you've begun to regain alertness after general anesthesia. You should be taken directly home and should plan to rest for the remainder of the day. During the first 24 to 48 hours of breast augmentation recovery, it is important that you stay hydrated. Doing so will help clear the anesthesia from your body. You will need to walk for a few minutes, a few times a day but should refrain from anything strenuous. When sleeping on your back, it is helpful to rest on two or more pillows. You may also want to place a pillow on each side to promote comfort and also to prevent rolling over.
- Your first week. Surgical side effects usually worsen two to three days after breast augmentation. During the first week of recovery, you may rest a lot, have little energy, and rely on prescription pain medication to mitigate soreness and tenderness. It's important to continue walking for five to 10 minutes several times a day to maintain proper blood flow through your legs. As your body engages in tissue repair, it's also important that you eat a healthy diet and stay well-hydrated.
- Your second and third weeks. As your discomfort lessens, you will gradually get back to some of your normal activities. Provided that your job is not physical in nature, you can expect to return to work 10 to 14 days after your breast augmentation. If your job does have some physical requirements, you may be off work for up to six weeks. You'll be very clear about Dr. Hunsaker's recommendations prior to scheduling your surgery. You cannot exercise, per se, but you might begin adding in some light cardio exercise at this point in your recovery. By now, chances are you'll be able to stay comfortable using over-the-counter pain medication.
- Weeks four through six. Most of your swelling, bruising, and discomfort will have resolved four to six weeks after breast augmentation. You'll have a post-op visit with Dr. Hunsaker around this time, at which point he will discuss what activities you can resume and which you may still need to avoid. Usually, it's necessary to wait at least six weeks to use the chest muscles.
- Your final results. After your first month post-op, the remainder of your breast augmentation recovery will revolve around "settling" and scar maturation. Once your incisions have healed fully, you may begin using scar cream or other treatments to help your body repair the minor incision wounds that were made during surgery. You may have a small amount of residual swelling after four to six weeks. As this goes down and your skin and tissue relax, your breast implants will settle into their natural, beautiful position.
Breast Augmentation At Cosmetique Cosmetic Surgery Center
In 18 years of business, our infection rate for breast implants is zero while published rates are as high as 2%. Our rate of capsular contracture, a complication resulting in the development of internal scar tissue, requiring surgical revision is less than 1%. Published rates for capsular contracture are about 2%-20%.
In the last two-and-a-half years over 96% of our breast augmentation patients have stopped needing any prescription pain medicine by the end of the second day. Additionally, nearly 50% stop by the end of the first day. Many patients use only Tylenol or Motrin after their procedure which has allowed them to plan their recovery over a long weekend. While every patient responds differently, and not every patient is a candidate for this approach, our method offers many advantages over more traditional approaches that require prolonged restrictions, recovery and pain.
Schedule Your Breast Augmentation Consultation In Miami!
If you wish to learn more about Breast Augmentation, or if you wish to schedule a personalized consultation with Dr. Robert Hunsaker, please call our office in Miami office at 305.279.4700. You can also reach us by filling out the appointment request form in our contact page. Dr. Hunsaker and his talented staff look forward to serving you!